Assessment of Lipid Profile among Women with Malignant and Non-malignant Breast Lesions in a Tertiary Hospital, Nigeria: A Pilot Study
Ajeigbe AK1, Omisore AD2, Akinde AO3, Makinde RA4, Ajose OA1
Abstract
Context: Breast cancer (BC) is the most common cancer in women. Reports suggest a possible relationship between lipids and BC; but the pattern of lipid profile in breast lesions is unknown.
Objective: to evaluate the lipid profile of women with BC and compare with benign breast lesions and controls.
Study design: Women (107) undergoing breast imaging in Ile-Ife were enrolled for this pilot study and stratified into BC, benign breast lesion (BBL), and controls. Information was obtained by a structured proforma followed by anthropometric measurements and the collection of blood for lipid assays (Total cholesterol [TC], triglyceride [TG], and high-density lipoprotein cholesterol [HDLc]) done on an autoanalyzer. Very low-density lipoprotein cholesterol (VLDLc) and Low-density lipoprotein cholesterol [LDLc] were calculated using the Friedewald equation. Mean serum concentrations of lipids were considered as normal, low, or high using standard cutoff. Data was analyzed by SPSS 22 using ANOVA. P < 0.05 was considered significant.
Results: Women with BC were significantly younger (51.6±10. 3 vs 62.5±9.5 and 59.0±12.7 years), and had significantly lower mean TG (1.1±0.4 vs 1.5±0.7 and 1.4±0.5 mmol/L), VLDLc (0.51±0.19 Vs 0.69±0.32 and 0.62±0.25 mmol/L) and HDLc (1.1±0.4 vs 1.4±0.3 and 1.5±0.3 mmol/L) than BBL and controls respectively. P < 0.05 However, women with BBL had significantly higher mean TC (6.0 ±1.2 vs 4.8±1.1 and 5.2±1.1 mmol/L), LDL-c (3.9±1.3 vs 3.2 ±0.9 and 3.2±1.0 mmol/L) respectively. P < 0.05
Conclusions: The BC group had hypoalphalipoproteinaemia while the BBL group had hypercholesterolemia. The dyslipidemia in BC is an independent cardiovascular risk factor.
Keywords: Breast cancer, Lipid, Women
Introduction
Globally, breast cancer (BC) is the most common malignancy in women with a huge burden in low-middle-income countries (LMIC) like Nigeria.1-2 The prevalence of BC in Nigeria is estimated to be 116 /100, 000 with 58% annual mortality.1-3 The overall survival of patients with BC is low in Nigeria.
The huge burden of BC in Nigeria is partly due to the late presentation from lack of awareness, poor attitude to screening, and the presence of aggressive tumour biology in the form of triple negative (ER -ve, PR -ve and HER -ve) variants.4-7 This is coupled with poor diagnostic tools necessitating the investigations of biological markers that are readily available in resource-limited environments like ours that can suggest or predict BC. An example of such is a marker of fat metabolism that may play a role in the pathogenesis of BC. Abnormal fat metabolism is also related to benign breast conditions, although it is of a lower threshold than BC.8
Apart from suggested physical parameters like weight, abdominal circumference, and body mass index that may be potential risks for the development of BC, markers of fat metabolism are potential targets for their link to BC.9 This is important because anthropometric parameters are limited by racial factors, menopausal state, and metabolic state.10-12 An example is the high body mass index (BMI) which appears protective of BC in premenopausal women but increases the BC risk in postmenopausal states.10 Also, individuals with normal BMI may be metabolically obese due to insulin resistance.10 Some other risks that are being assessed include menopausal status, age at menarche, and parity. Since these physical and physiological variables are associated with fat metabolism, it is important to explore the role of lipoproteins among women diagnosed with BC and those with benign breast lesions in comparison with apparently healthy women. This appears to be a more objective way of assessing the relationship between BC and fat metabolism than anthropometry.
Subject/Materials and Methods
This was a cross-sectional preliminary study involving women on routine BC screening at the radiology department of Obafemi Awolowo University Teaching Hospital (OAUTH), Ile-Ife, and those referred to OAUTH on account of BC between January 2022 and December 2023. Those with chronic illness (diabetes mellitus, hypertension, chronic liver disease, and chronic kidney disease), lipid-lowering drugs, hormone replacement therapy, and diagnosed BC on treatment were excluded from the study. Ethical approval was obtained from the ethics committee of OAUTH, Ile-Ife. A semi-structured questionnaire was used to obtain information on demographic data, social background, and past medical history. This was followed by measurements of weight and height using a weighing scale and stadiometer recorded in kilogram (kg) and meter (m) respectively. Waist and hip circumference were measured using a tape measure in centimeters (cm). Body mass index and waist-hip ratio were calculated using the measured anthropometry. Five (5) ml of random13 (indicated for routine assessment and to prevent loss to follow-up) venous blood specimens were collected from consenting women into a plain bottle for lipid assay (TC, TG, HDLc) using Cobas c311 (Roche Diagnostics, Germany). Blood specimens were allowed to clot and centrifuged at 1500 g for 10 minutes. The supernatant serum was harvested by Pasteur’s pipette into an Eppendorf and analyzed immediately at the Postgraduate/Metabolic Research Unit of OAUTH, Ile-Ife. Results for LDLc and VLDLc were calculated using Friedewald’s equation14, LDLc (mmol/L) = TC – (TG/2.2+HDLc); VLDLc – TG/2.2. Mean serum concentrations of lipids were considered as normal, low, or high using the cutoff values from the authors’ local laboratory (TC = 5.1 mmol/l, TG = 1.7 mmol/L, LDLc = 3.3, HDLc >1.1 mmol/L). All women had breast imaging (ultrasound and mammography) done and those with suspicious breast lesions progressed to have image-guided biopsy for histology. Women were further stratified into 2 groups based on their histology report viz breast cancer (BC), and benign breast lesion (BBL) while those with normal imaging served as controls. Those with malignant or benign breast lesions were navigated into a surgical oncology clinic for management. Data were entered into an Excel spreadsheet and analyzed by SPSS 22 using ANOVA. P < 0.05 were considered significant.
Results
This study found that the mean age (51.6±10.3 years) of women in the BC subgroup was lower than those with BBL and controls (62.5±9.5 and 59.0±12.7 years) respectively. This was statistically significant. P < 0.05. Table 1
The mean BMI of all the study participants was above normal (18.5 – 24.9 Kg/m2) reference. Although, the BC subgroup had a lower BMI (26.8±4.9 Kg/m2) compared to those with BBL (29.2±4.6 Kg/m2) and controls (28.4±5.6 Kg/m2). This was however not statistically significant. Similar to the BMI, the mean waist-to-hip ratio (WHR) for those with BBL was found to be higher (0.98±0.09) than the BC and controls (0.91+0.07 and 0.90 ±0.08) respectively. This was statistically significant. P <0.05. Table 1
Table 1: Demography and Anthropometry of study participants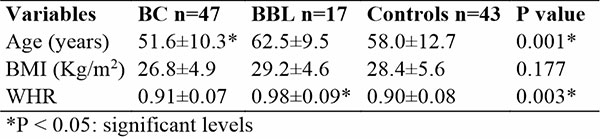
Women in the BC subgroup had lower mean lipid parameters (TC, TG, LDLc, VLDLc and HDLc) than the controls and those in the BBL subgroup, however, statistical significance was found for only TG (1.1±0.4 Vs 1.4 ± 0.5 and 1.5 ± 0.7 mmol/L), VLDLc (0.51±0.19 Vs 0.62±0.25 and 0.69±0.32 mmol/L) and HDLc (1.1±0.4 Vs 1.5±0.3 and 1.4±0.3 mmol/L) respectively. P < 0.05. Participants in the BBL subgroup had higher mean lipid values (TC:6.0±1.2; TG: 1.5±0.7; LDLc:3.9±1.3; and VLDLc: 0.69±0.62 mmol/L) than the controls and BC except the HDL-c which was slightly lower than the controls (1.4±0.3 and 1.5±0.7 mmol/L). Significant levels were found for only TC (6.0±1.2 mmol/L Vs 5.2 ±1.1 and 4.8 ±1.1) and LDLc (3.9±1.3 Vs 3.2±1.0 and 3.2±0.9 mmol/L) respectively. P < 0.05. Table 2.
Table 2: Comparison of Lipid Parameters among Study Participants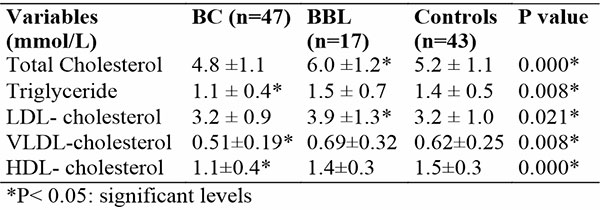
Table 3, shows that more women with BBL had elevated TC (88.2%), LDLc (76.5%), and HDLc (82.4%) while controls had only elevated TC (51.2%) and HDLc (90.7%). Only TC and LDLc showed significant levels (P < 0.05). However, a lower proportion of women with BC had elevated TC (40.4%), (10.6%), LDLc (40.4%), and HDLc (36.2%). The proportion of women with low HDLc in the BC group was statistically significant. P < 0.001.
Table 3: Proportion of Lipid Parameters based on Cut-off Values among Study Participants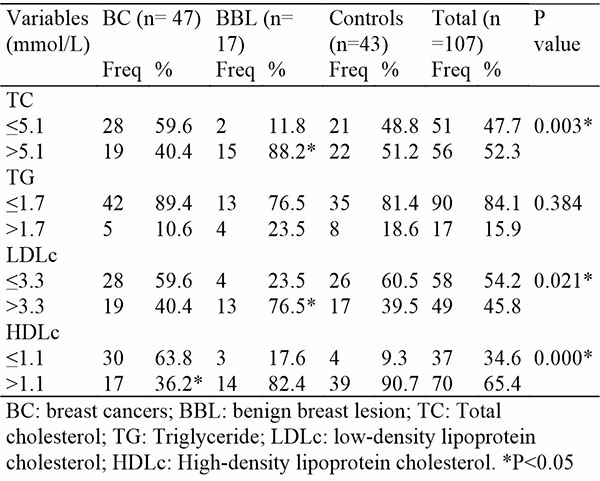
Discussion
Breast cancer is the most common cancer in women globally with a huge burden in Africa. Women in Nigeria with BC usually present in the late stages of the disease partly due to sociocultural and environmental factors. Thus, the poor outcomes of BC in Nigeria. The prevalence of BC in Nigeria is 22.7% with a mortality rate as high as 58%.2,3,7 Previous studies reported a potential relationship between lipid parameters and BC.9,10,15 This is with the possibility of deploying lipid parameters for routine prediction of BC. Also, to provide evidence of cardiovascular risk in BC and the need for cardiovascular support among survivors of BC8,10. This will improve the overall well-being of BC patients and survivors.
Similar to previous reports, this pilot study found that women with BC were younger than those with BBL and controls.8,10,14 A study conducted in Ibadan, Nigeria on lipid profile assessment among BC patients and controls showed that women with BC had a mean age of 52.1±12.0 years which is comparable to 51.6±10.3 years found in this study.10 Another study in Qatar reported the mean age of women with BC as 42.0±9.2 years.15 While a study in Ethiopia reported a much lower mean age of 37.65±14.3 years for women with BC.8 Meanwhile, in this study, we observed that BBL was more common among older women.8,15 This finding is different from the report of a study conducted in Ethiopia on lipid profile of women with BC and benign breast lump. Women with benign breast conditions were younger (33.34±10.66 years) than those with BC (37.65 ±14.3 years).8 This difference may be due to the younger age group recruited for that study. Probably, the women in that environment had a better screening culture and presented earlier than those in this study.
The mean BMI for the subgroups and controls in this study showed that the participants were overweight whether or not a breast lesion was present. Although those with BBL had a higher BMI, this was not statistically significant. This may suggest that BMI is not a strong tool for assessing breast conditions in women. This finding aligns with a previous report that BMI is a limited tool for the assessment of fat metabolism.10
Similar to higher BMI, a higher WHR which was statistically significant was observed for the BBL subgroup than BC and controls. This observation suggests abdominal obesity among BBL and implies that WHR is a better anthropometric tool for assessing fat metabolism, especially in this cohort of patients. In addition, it was observed that as the age increases, the BMI also increases, this however, was not the same with the WHR. Thus, suggests that the WHR may not be influenced by overall body fat and therefore WHR may be regarded as an independent risk factor for breast condition.
The lipid parameters from this study showed that the BC participants had lower values for all the components compared to the controls and the BBL subgroup. However, significant levels were found for only the TG, VLDLc, and HDLc. This supports an earlier report by Li et. al. in China where it was observed that women with BC had lower values for all the lipid parameters compared with controls.16 This observation is probably linked to the lower BMI. Thus, reflecting the wasting nature of the disease from an increased catabolic state coupled with anorexia. Persons with malignancy often experience weight loss, poor appetite, and cachexia driven by cytokines produced by the cancerous cells. These cytokines may also be involved in lipolysis and lipid metabolism. Studies have shown that cancer cells elaborate lipogenesis drawing upon the fat stores and modulating the Apolipoproteins resulting in reduced HDLc.17 Therefore, lower lipid values found in BC may not necessarily be an optimal lipid state. Rather a component of the pathologic process indicating the severity of the disease in BC. Although, this present report is a pilot, post-treatment follow-up and reassessment of the lipids will provide a piece of robust information about their lipid status. This is because the evaluation of serum lipids is an important strategy for the effective management of BC and comorbidities.18
The mean TG level for the BC subgroup was within the normal reference of < 1.7 mmol/L despite a random blood specimen being used in the analysis of lipids. Suggesting a reduced TG-rich lipid store. Probably due to a higher demand for energy by the cancerous cells utilizing the TG through lipolysis and not necessarily, an optimal physiologic state. However, the HDLc was observed to be lower than the reference of > 1.1 mmol/L suggesting hypoalphalipoproteinaenamia. Whether the observed HDLc result is due to a menopausal state with reduced estrogen level or depletion of HDLc from increased dependence on lipid metabolism by cancerous cells is unknown. However, the scavenger receptor class B1 in human breast tissue has been reported to be an HDLc receptor that mediates the uptake of cholesterol.17 Similar to this finding, previous studies reported lower HDLc among BC patients. In a study in Qatar, HDLc of 0.99±0.18 mmol was reported for women with BC.8 Similarly, a low HDLc (39.6±6.98 mg/dl) was reported from a study conducted in Bangladesh, India.19 In another study by Cedo et. al., abnormal HDLc and LDLc were associated with BC and their normal levels were prognostic markers for breast tumour.17 However, a study from Ibadan, Nigeria, reported that higher TC, TG, and HDL were found for women with BC than the controls.10 This present study only found significantly lower HDLc among the women with BC compared with women having BBL and controls.
In contrast to lipid findings among the BC women, those with BBL had higher TC and LDLc suggesting hypercholesterolaemia, a dyslipipidaemic state. This is supported by an earlier report that an abnormal lipid profile (hyperlipidaemia) is associated with benign breast disease.20 A previous study reported higher mean levels of TG (211.7±82.924 mg/dL) for the BC group than the controls and benign conditions which is in contrast to the findings from this study.8 The cohort of participants in that study was much younger than those recruited for this study. However, the mean HDLc (38.2026 ±7.442 mg/dL) for the BC group in that study was also found to be lower in support of the findings from this study.8
Similar to the mean values for the lipids, this study observed that a higher proportion of women with BBL and controls had elevated TC, TG, LDLc, and HDLc. While higher proportions of those in the BC group had lower values. This finding is different from previous reports of a higher proportion of women with BC had elevated TC, TG, and LDLc.8,21 A study from Bangladesh reported elevated TC, TG, and LDLc in controls (30 %, 16%, and 44%), benign breast disease (32%, 18%, and 50%), and higher proportions for breast cancer (75%, 52%, and 82%) respectively.21 Likewise, another study from Ethiopia reported that higher proportion of women with malignant breast tumour (17.39%, 69.56%, and 26.08%) had elevated TC, TG, and LDLc than controls (3.32%, 46,1%, 12.08%), and benign breast lump (11.76%, 54.4%, 11.76%).8 However, more women (62% and 60.87%) with BC in Bangladesh and Ethiopia respectively were reported to have low HDLc in support of the findings in this study.8,21
This study observed that the BC subgroup and the controls had comparable lipid profile parameters in TC and LDLc which were optimal. This is against the BBL subgroup that had hypercholesterolaemia while the BC group had hypoalphalipoproteinaemia. A report from India showed no statistical difference in the mean concentrations of TC of BC and controls similar to our findings.21 Another study also reported that TC was higher in BBL than the controls further supporting the findings in this study.8 Therefore, this study found that the mean levels of TC and LDLc for the women with BBL suggest hypercholesterolaemia while the mean levels of HDLc for the BC subgroup suggest hypoalphalipoproteinaemia. Hence, both benign and malignant breast conditions may be associated with dyslipidaemia for which support is needed.
The hypoalphalipoproteinaemia found in the BC participants is consistent with past reports.8,10,15,21 It is a singular cardiovascular risk suggesting a need to optimize cardiovascular function among BC patients. This may be necessary to improve the overall well-being of survivors of BC and improve outcomes.
Conclusion: This study found abnormal lipid parameters in the form of hypercholesterolaemia in women with BBL and hypoalphalipoproteinemia in those with BC. Thus, dyslipidaemias exist in women with breast lesions irrespective of their malignant status. Hypoalphalipoproteinaemia is an independent risk for cardiovascular disease and women with BC may be at risk of developing cardiovascular disease. Hence, support for their cardiovascular function.
Recommendation: It is recommended that lipid profile assay should be part of routine breast cancer screening.
Limitation: This study is limited because the influence of age and glycaemic state on serum lipids was not assessed.
Ethics approval and consent to participate: This study was approved by the ethics review board of OAUTH, Ile-Ife.
Consent for publication: all participants in this study gave written informed consent to be enrolled in this study
Conflict of interest: None
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68:394- 424.
- International Agency for Research on Cancer. GLOBOCAN 2008. Section of Cancer Information. Lyon, France: International Agency for Research on Cancer; 2010.
- Newman LA, Alfonso AE. Age related differences in breast cancer stage at diagnosis between black and white patients in an urban community hospital. Ann Surg Oncol. 1997; 4:655-662.
- Adebamawo CA, Ajayi OO. Breast Cancer in Nigeria. West Afr J Med. 2000; 10:179-191.
- Edino ST, Ochicha O, Alhassan S, Mohammed AZ, Ajayi OO. Clinico-Pathological Review of Breast Cancer in Kano. Nigerian Journal of Surgery. 2000; 7:70-75.
- Agboola AJ, Musa AA, Wanangwa N, Abdel-Fatah T, Nolan CC, Ayoade BA, et al. Molecular characteristics and prognostic features of breast cancer in Nigerian compared with UK women. Breast Cancer Res Treat 2012; 135:555-69.
- Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, Friedman ER, Slingerland JM. Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention. CA Cancer J Clin 2017; 67:378-97.
- Kumie, T. Melak, H. B. W Baynes. The Association of Serum Lipid Levels with Breast Cancer Risks Among Women with Breast Cancer at Felege Hiwot Comprehensive Hospital, Northwest, Ethiopia. Breast Cancer: Targets and Therapy. 2020. 279 – 287. DOI: 10.2147/BCTT.S279291.
- Omolara A. Fatiregun, Temiloluwa Oluokun, Nwamaka N. Lasebikan, Emmanuella Nwachukwu, Abiola A. Ibraheem, and Olufunmilayo Olopade. Breast Cancer Research to Support Evidence-Based Medicine in Nigeria: A Review of the Literature. JCO Global Oncology. 2021:7: 384 -390.
- Olabumuyi A.A., Abdus-Salam A.A., Ogunnorin B.O., Kuti M.A. Lipid Profile in Breast Cancer Patients: A Case-Control Study Done at a Public Tertiary Hospital in Ibadan Nigeria. Niger J Med. 2021: 30; 519 – 525.
- Chandran U, Hirshfield KM, Bandera EV. The role of anthropometric and nutritional factors on breast cancer risk in African-American women. Public Health Nutr 2012;15:738-48.
- Park YM, Fung TT, Steck SE, Zhang J, Hazlett LJ, Han K, et al. Diet Quality and Mortality Risk in Metabolically Obese Normal-Weight Adults. Mayo Clin Proc 2016;91:1372-83.
- Olumese FE, Idogun ES. Effect of Diet on Serum Lipid Profile in Healthy Nigerians. Annals of Tropical Pathology. 2016. 7(1). 17-26.
- Kirkwood BR, Sterne JA. Calculation of required sample size. In: Essential Medical Statistics. 2nd ed. Oxford: Blackwell Publishing Ltd.; 2003. p. 413-28.
- Al Ghatam G, El Mistiri M, Belbraouet S. Serum Lipid Profile and Breast Carcinogenesis among Qatari Young Women. Clin Oncol. 2023; 8; 2025.
- Li X, Liu Z, Wu Y, Wu H, Dai W, Arshad B et al. Status of lipid and lipoprotein in female breast cancer patients at initial diagnosis and during chemotherapy. Lipids in Health and Disease. 2018. 17:91: https://doi.org/10.1186/s12944-018-0745-1.
- Cedo L, Reddy S.T., Mato E, Blanco-Vaca F., Escola-Gil JC. HDL and LDL: Potential new players in breast cancer development. J Clin Med. 2019: 8(6): 853. DOI: 10.33901jcm8060853.
- Coates A, Ahern T, Borgyquist S, Colleoni M, Debled M, Ejlertsen B. Editors. Cholesterol Medication use and breast cancer outcome in the BIG 1- 198 study. Am Soc Clin Oncol. 2017: 35:11:1179 -1188.
- Prabakar M.S., Nithyaraj P., Reshma S., Loganathan K., Palani V. A clinical study of serum lipid profile in benign breast disease in a tertiary care hospital. Int Surgery Journal. 2019. 6(9). 3162-3164. DOI: http://dx.doi.org/10.18203/2349-2902.isj20193603
- Chowdhury F.A., Islam Md F., Prova M.T., Khatun M, Shamin I, Islam K.M. et al. Association of hyperlipidemia with breast cancer in Bangladeshi women. Lipids in Health and Disease. 2021. 20:52. DOI: 10.1186/s12944-021-01480-2
- Laamir FZ, Otmani A, Ahid S, Barkat A. Lipid profile among Morrocan overweight women and breast cancer: a case-control study. Int J Gen Med. 2013: 6: 439.