Exploring patient-centered barriers to hemophilia care in Southern Nigeria: Study Protocol
Kelechi Eguzo1, Timothy Ekwere2, Becky Kirikareye1, Helen Okoye3, Peace Egharevba4, Chukwuemeka Oluoha5
Abstract
Background: Hemophilia is a rare, inherited bleeding disorder which affects about three out of every one million Nigerians. Evidence shows that most PLwH in Nigeria do not know enough about the condition and face some barriers. The goal of this paper is to describe the study protocol used to develop and validate tools that would explore the barriers and knowledge gaps related to hemophilia, as a part of a larger needs assessment study.
Methods: This exploratory mixed methods study used surveys and focus group discussions (FGD). Participants included PLwH/caregivers (i.e. PLwH) and healthcare professionals (HCP) in southern Nigeria. Study instruments were tested for validity and reliability by a purposive sample of target participants. Quantitative data was analyzed using descriptive and inferential, while thematic analysis was used for qualitative data.
Results: Thirty-seven individuals participated in the pre-testing of both survey tools (HCP – 18; PLwH – 19). For the PLwH, their average age was 37±19 years and had been associated with hemophilia for about 10 years (IQR: 2,12). Whereas the average years in practice for the HCPs was 13±6 years and had encountered about 20 patients (IQR: 6, 32) with hematologic disorders in the previous month. A Cronbach's alpha of 0.92 (PLwH Survey) and 0.91 (HCP survey) was obtained for the surveys, indicating excellent internal consistency. FCD revealed concerns about the introduction of the surveys, their length, and time required to complete the questions. The surveys were revised as needed.
Conclusion: The tools developed to evaluate barriers and knowledge about hemophilia by PLwH and HCPs were valid, appropriate and reliable. It is important therefore to deploy the instrument on a larger scale with the expectation that findings from that study would be valid, reliable and useful in planning future interventions regarding hemophilia.
Keywords: Hematologic Diseases, Needs Assessment, Hemophilia, Focus Groups, Nigeria, validity and reliability
Introduction
Hemophilia is a relatively rare, X-linked inherited bleeding disorder caused by a deficiency of functional coagulation factor VIII (hemophilia A) or factor IX (hemophilia B). Clinically, hemophilia can be classified as severe hemophilia (defined as a residual clotting factor activity of less than 1%), moderate-severe hemophilia (between 1% and 5%), and mild hemophilia (between 6% and 40%).1 A 2021 survey identified 630 people living with hemophilia (‘PLwH’) in Nigeria, with a national population of 206,000,000, giving a prevalence of 3 cases per 1 million.2 People living with severe hemophilia present with spontaneous joint and muscle bleedings leading to joint damage, resulting in higher emergency room visits and hospitalization compared to the rest of the population.3 The treatment options for hemophilia includes regular infusion with coagulation factor concentrates or nonfactor blood products. Almost 40% of PLwH in Nigeria were rated to have poor health status as evidence by research which shows that almost 50% of this population in Nigeria were hospitalized for acute exacerbation within a 12 month period.4,5 Despite this trend, evidence shows that most people living with hemophilia in Nigeria as well as many healthcare providers do not know enough about the condition.3
Previous research identified barriers that are faced by hemophilia patients globally. These are classified as educational barriers, such as lack of awareness among patients regarding the disease symptoms and course as well as inconvenience and scheduling barriers, where caregiver may not be available as needed. Financial barriers often exists, where patients can neither pay for the clotting factors nor the logistics needed to receive donated clotting factor products as well as psychosocial barriers.6 However, there is no Nigerian research to show the extent of patients’ knowledge, attitude, care patterns, and barriers related to hemophilia. This is further complicated by the absence of a validated tool to evaluate barriers faced by patients with hemophilia in Nigeria.
The overarching goal of this paper is to describe the study protocol used to develop and validate a tool that would be used to explore the barriers and knowledge gaps related to hemophilia. It is a part of a larger needs assessment study on hemophilia in southern Nigeria. The objectives of the larger project are to:
- Ascertain knowledge about, attitudes toward, and behaviors associated with key prevention activities, as well as barriers to accessing novel treatments among PLwH and caregivers
- Understand the self-reported health status of PLwH in Nigeria
- Evaluate the knowledge gaps, practice patterns, and barriers experienced by local clinicians regarding the management of hemophilia
- Proffer solutions to the barriers to learning about the clinical needs for PLwH in Nigeria.
No previous studies have evaluated the knowledge of hemophilia among PLwH and caregivers in Nigeria.
Methods
Study Design
This was an exploratory mixed methods study, involving surveys and focus group discussions (FGD), to evaluate the utility of new tools designed to test the hemophilia-related knowledge, attitudes, care patterns, and barriers PLwH encounter (Objective #1). This considered the perspectives of the PLwH/caregivers and the HCPs. This design has been used in the past to explore multiple perspectives related to a health outcome in Nigeria.7 The surveys had a mix of structured and open-ended questions which assessed (a) knowledge of hemophilia; (b) compliance with recommended prevention behaviors; (c) self-management of hemophilia; (d) knowledge, beliefs, and practices related to dealing with joint disease and infections; and (e) self-reported evaluation of health status (Objective #2). The surveys were deployed online, through telephone, and on paper at hospitals that manage hemophilia. In addition to answering the survey questions, the participants were also required to rate each question for clarity and relevance to the overarching research goals.
After completing the survey, interested participants were requested to join the FGD to further explore the depth of their understanding of the questions, the barriers they faced and how these barriers might be overcome. The FGD were recorded and transcribed to further analyze the data. Regarding the knowledge and competence of local healthcare providers (Objective #3), an online self-administered survey with an embedded quiz was deployed to a purposive sample of consenting HCP. Both surveys included a self-grading quiz to determine the knowledge score of the participants. A section of the survey evaluated the providers’ practice patterns, self-reported learning needs, and perceived barriers to providing care to PLwH. Focus group discussions with HCPs were used to explore the usefulness of some evidence-based approaches to improving provider knowledge and self-management procedure for the PLwH.
Study Setting and participants
This study was focused on southern Nigeria, with emphasis on the southeast and south-south regions. The target participants in this project include the following groups:
- People living with hemophilia and their caregivers: These include family members of the PLwH, with age ranging between 18 and 60 years
- Healthcare providers (HCP) involved in managing hemophilia: these include physicians, nurses, pharmacists, community health workers and laboratory scientists
For the larger study, a minimum sample size of 139 participants is required to determine the mean hemophilia-related knowledge score of HCP, with 0.5 precision and 95% confidence interval. It is estimated that more than 150 HCP and 120 PLwH/caregivers will be involved in the project. However, a purposive sample of target participants was required to test the study instruments. Participants were recruited through patient associations (including the Hemophilia Foundation of Nigeria) and hospitals that manage hemophilia in the study setting. Targeted social media adverts and posts in relevant WhatsApp groups were also used to recruit participants within the region. Consenting participants were required to complete the online survey.
Study Instruments Development and Testing
The instruments used in this mixed methods study were developed through a review of relevant literature. Different instruments were developed for each group of participants, i.e. PLwH and HCP. The PLwH survey had 25 items that were grouped into demographics, educational barriers, technical barriers, financial barriers, perception of stigma and knowledge quiz. Similarly, the HCP survey had 25 items that were grouped into demographics, educational barriers, technical barriers, financial barriers, perception of stigma and knowledge quiz. After the instruments were developed, they were circulated among a panel of eight experts (including PLwH) to evaluate their face validity. Face validity is the subjective estimation regarding the clarity of an instrument.8 A survey tool with high face validity means that the wording of the content are clear to the experts, and that it measures what it is intended to measure.8 After the expert panel was satisfied with the face validity, the surveys were tested for content validity. The content validity essentially determines how well a tool encompasses all relevant parts of the concept it aims to measure.8 The expert panel, guided by a review of relevant literature, ensured the content validity by establishing the connections between survey items and the concept of barriers. They achieved this by independently evaluating electronic versions of the survey tools and indicating if the wording and structure of the tools appropriately represented the intended measure.
Furthermore, the reliability of the instrument was tested. Reliability shows the consistency of the scores acquired from the instrument. There are different ways to estimate the reliability of a survey tool such as: Test-Retest, Parallel-Forms, and Inter-Rater reliability. The inter-rater reliability is estimated by comparing the scores of two or more independent individuals on the responses of the participants to the instrument; the comparison is used to determine whether the raters’ estimates are consistent.8 For this study, a sample of ten eligible participants from each group (PLwH/caregivers and HCP) tested each survey. Each survey question was evaluated using a 5-point Likert-like scale for clarity. The Cronbach's alpha was computed for each of the survey tools. Cronbach's alpha is a measure used to assess the reliability, or internal consistency, of a set of scale or test items. In other words, the reliability of any given measurement refers to the extent to which it is a consistent measure of a concept, and Cronbach’s alpha is one way of measuring the strength of that consistency.8 The interpretation of Cronbach’s alpha ranges from poor (a<0.5) through acceptable (a>0.7) to excellent (a>0.9).
Ethical consideration
Ethics approval for this project was obtained from the Human Research Ethics Committee at the Abia State Ministry of Health as well as the Ethics Committee at Hemophilia Foundation of Nigeria. This research did not collect personal health information, outside of the study instrument. All potential participants were informed of their right to withdraw from the study before joining the course. Research data was managed according to international standards.
Although individuals who participated in the focus group discussion did not remain anonymous, pseudonyms were assigned to protect their identities. The project team ensured high ethical standards, including respect for human dignity; respect for free and informed consent; respect for vulnerable persons; respect for privacy and confidentiality; respect for justice and inclusiveness; and, balancing harms and benefits by minimizing harm and maximizing benefits
Data Analysis
Data analysis involved a variety of statistical tests. Continuous data was analyzed using descriptive statistics (mean, standard deviations, and proportion) and inferential statistics (student T-tests, analysis of variance, and non-parametric tests) to highlight and compare knowledge levels and Likert-like ratings of disease severity. Chi-square tests was used to categorize and compare attitude, practice, and self-care patterns, as well as perceptions of barriers. Multiple regression was used to determine predictors of poor outcomes, with the intent of prioritizing interventions for different patient demographics.
Preliminary Results and Discussion
A total of thirty-seven (37) individuals participated in the pre-testing of both survey tools (HCP – 18; PLwH – 19). For the PLwH/caregivers, their average age was 37±19 years and had been associated with hemophilia for about 10 years (IQR: 2,12). Whereas the average years in practice for the HCPs was 13±6 years and had encountered about 20 patients (IQR: 6, 32) with hematologic disorders in the previous month. Table 1 shows the demographics of the participants in the pretesting.
Results
Reliability Testing for Surveys
The degree of internal uniformity among the items, namely the correlation between the items and the five related domains, was expressed by Cronbach’s coefficient. The following rules of thumb for Cronbach's alpha were: >0.9 Excellent; >0.8 Good; >0.7 Acceptable; >0.6 Questionable; > 0.5 Poor; and <0.5 Unacceptable.9 Cronbach's alpha were computed for each survey and its associated quiz. As shown in Table 2, a Cronbach's alpha of 0.92 (Patient Survey) and 0.91 (HCP survey) was obtained for the surveys, indicating excellent internal consistency.
Table 1: Demographic Characteristics of pre-testing participants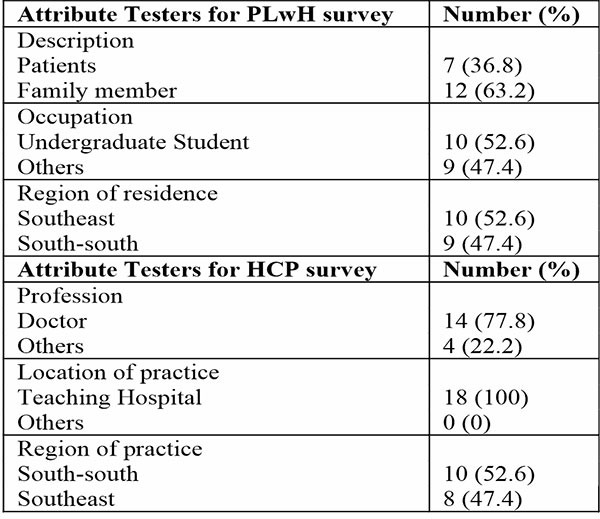
Table 2: Reliability analysis for each survey instrument using Cronbach Alpha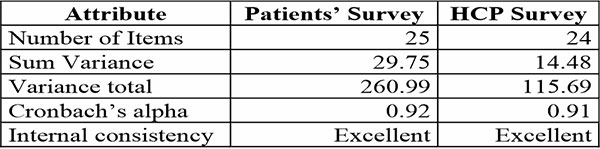
The analysis from reliability testing showed that the questions were clear to the participants, and that the survey was well suited to measure what it was intended to test. The average quiz performance from the patients/caregivers was 60% and that of the HCP was 60% (out of 20 questions). This demonstrated that the quiz questions were fair. Furthermore, the participants were asked to express interest in learning more about hemophilia through a support group for patients and an online course for HCPs. About 95% (18/19) of patients/caregivers and 83% (15/18) of HCPs gave positive responses. This suggests that interest regarding hemophilia within the study population might be high.
Qualitative Feedback from Instrument Testing
Focus group discussions involving participants of the pretesting revealed concerns about the introduction of the surveys. According to a reviewer, “the intro assumes that everybody, including the healthcare professionals, knows what haemophilia is even at the face value. It will help to give a one sentence introduction of what the disease is, at face value then followed by other things leading to the survey questions”. This led to the revision of the survey introduction, without altering the order or number of questions. Also, there was a concern regarding number of questions and time commitment required. “The average time it took to complete the surveys was a consistent question from the participants especially the HCP in the pretesting survey. If we can find a way to reduce the number of questions while still retaining the content of the survey, it will make the larger participation easier”. Due to the scope of the surveys, a consensus was reached that it was not expedient to reduce the number of questions.
Conclusion
Although hemophilia is a rare disease it affects enough people in Nigeria to warrant increasing attention. The tools developed to evaluate barriers and knowledge about hemophilia by PLwH and HCPs were valid, appropriate and reliable. It is important therefore to deploy the instrument at a larger scale with the expectation that findings from that study would be valid, reliable and useful in planning future interventions regarding hemophilia.
References
- BRANDS, M. et al. Patients’ and health care providers’ perspectives on quality of hemophilia care in the Netherlands: a questionnaire and interview study. Research and practice in thrombosis and haemostasis, v. 7, n. 4, 2023. Disponível em: < http://dx.doi.org/ >.
- STONEBRAKER, J. et al. Report on the Annual Global Survey 2020. World Federation of Hemophilia. Montreal, p.96. 2021
- OKOYE, H. et al. Health Status of Persons with Hemophilia: A Pilot Survey from a Resource-Constrained Country. Niger Med J, v. 60, n. 2, p. 87-91, 2019. Disponível em: < https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6688393/ >.
- BUCKNER, T. et al. Health care resource utilization and cost burden of hemophilia B in the United States. Blood Adv, v. 5, n. 7, p. 1954-1962, 2021. Disponível em: < https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8045501/ >.
- BOLARINWA, A. et al. Inhibitor Epidemiology among People Living with Haemophilia A in South West Nigeria - ISTH Congress Abstracts. Res Pract Thromb Haemost., v. 4, n. Suppl 1, 2020. Disponível em: < https://abstracts.isth.org/abstract/inhibitor-epidemiology-among-people-living-with-haemophilia-a-in-south-west-nigeria/ >.
- SAXENA, K. Barriers and perceived limitations to early treatment of hemophilia. J Blood Med, v. 4, p. 49-56, 2013. Disponível em: < https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3660133/#:~:text=Barriers%20and%20perceived%20limitations%20to%20early%20treatment%20in%20patients%20with,financial%20barriers%2C%20and%20psychosocial%20barriers. >.
- EGUZO, K. N. et al. Using multiple perspectives analysis to propose state cancer control policy in Abia State, Nigeria. Journal of Clinical Oncology, v. 38, p. e14132-e14132, 2020-05-25 2020. Disponível em: < https://ascopubs.org/doi/abs/10.1200/JCO.2020.38.15_suppl.e14132 >.
- OKTAVIA, R. et al. Assessing the validity and reliability of questionnaires on the implementation of Indonesian curriculum K-13 in STEM education. Journal of physics: Conference series, v. 1088, n. 1, 2018-09-01 2018. Disponível em: < https://iopscience.iop.org/article/10.1088/1742-6596/1088/1/012014/meta >.
- LANCE, C.; BUTTS, M.; MICHELS, L. The sources of four commonly reported cutoff criteria: What did they really say? Organizational research methods., v. 9, n. 2, p. 202-20, 2006-04-01 2006. Disponível em: < https://journals.sagepub.com/doi/10.1177/1094428105284919 >.