HBME-1 expression by thyroid neoplasms in a Nigerian tertiary health Centre
Soremekun A I, Olaofe O O, Komolafe A O
Abstract
Aim: The aim of this study is to review the histopathological diagnosis of thyroid neoplasms seen in OAUTHC Ile Ife using HBME-1 immunohistochemical marker.
Materials and methods: We studied all thyroid neoplasms diagnosed in the Department of Morbid Anatomy and Forensic Medicine OAUTHC, Ile-Ife from 1st July 1996 to 30th June 2016. We noted the age, sex of the patients, nature of histological diagnosis (benign and malignant) and the specific histologic diagnosis. We evaluated HBME-1 immunostain grade (0, 1+, 2+, 3+, 4+) for all the tumours.
Results: We studied only fifty-six cases (all neoplastic thyroid diseases). Sixteen (28.6%) and forty (71.4%) cases of the cases were benign and malignant, respectively. The Malignant thyroid neoplasms were 2.5 times more frequent than the benign thyroid lesions. There is a significant statistical relationship between HBME-1 expression and the nature of the neoplastic cases (p = 0.001). The sensitivity of HBME-1 in distinguishing benign from malignant thyroid neoplasms in this study was 77.5%, the specificity was 87.5%, the positive predictive value (PPV) was 93.3% and the negative predictive value (NPV) was 60.1%.
Conclusion: There is a significant statistical association between HBME-1 and the nature of thyroid neoplasms. HBME-1 can be used as an added tool in distinguishing malignant thyroid neoplasms from the benign tumours.
Keywords: Thyroid, Neoplasm, Immunohistochemistry, Antibody
Introduction
Thyroid neoplasms are the most common neoplasm of the endocrine glands.1 The benign neoplasms are far more common than the malignant ones by ratio of 10:1 in the United States,1 3:1 in Addis Ababa, Ethiopia2 and Zaria in Nigeria3, and by 4:1 in Accra Ghana4 and Ibadan Nigeria.5 An increase in the incidence of thyroid carcinoma has been noted in recent years and papillary thyroid carcinoma is by far the most common type of thyroid malignancies.6
Diagnosis of thyroid lesions have been challenging to histopathologists. Several studies tried to identify antibodies that could improve the ability of pathologists to differentiate malignant from benign thyroid tumours. A variety of biomarkers have been studied in relation to thyroid neoplasm.7-9
Hector Battifora Mesothelial-1 (HBME-1) is a membrane antigen found in the microvilli of mesothelial cells, normal tracheal epithelium, and adenocarcinoma of the lung, pancreas, and breast.10 Follicular epithelial cells in normal thyroid tissue are usually negative for HBME-1.7 HBME-1, one of the most frequent antibody evaluated by investigators, is described to have a strong and widespread expression in malignant thyroid tumours.7 HBME-1, in the thyroid, stains mostly follicular-derived malignant tumours, including well-differentiated and poorly differentiated carcinomas.8
HBME-1 has been described by several researchers to be a useful marker of malignancy in thyroid nodules.9,11 Overexpression of HBME-1 has been demonstrated in malignant thyroid neoplasms, especially papillary thyroid carcinomas (PTC). However, Hurthle cell carcinomas are usually negative for HBME-1 antigens.7
Studies have shown a high sensitivity and specificity of HBME-1 in diagnosis of malignant tumour of the thyroid gland. Liu et al found the overall sensitivity of HBME-1 for thyroid malignancy to be 78.8%. The sensitivity of papillary thyroid carcinomas and follicular thyroid carcinomas were found to be 87.3% and 65.2% respectively.7 This has made some researchers to recommend the use of HBME-1 antibody as an added tool in diagnosis of thyroid malignancies.
HBME-1 is expressed in some non-malignant lesions of the thyroid. This includes benign neoplasms and inflammatory lesions. Focal expression of HBME-1 was well-known in benign thyroid lesions such as follicular adenoma, nodular goitre, and lymphocytic thyroiditis.7 Although it is expressed in these non-malignant lesions, its staining pattern differs from the strong and diffuse feature seen in malignant lesions.
The aim of this study is to review the histopathological diagnosis of thyroid neoplasms seen in OAUTHC Ile Ife using HBME-1 immunohistochemical marker and to describe their immunohistochemical profiles using the HBME–1.
Materials and methods
Study Design and Setting
We did a cross-sectional study in the Department of Morbid Anatomy and Forensic Medicine, Obafemi Awolowo University Teaching Hospital Complex (OAUTHC), Ile-Ife. OAUTHC is a tertiary health care center in southwestern Nigeria.
Participants
We studied all thyroid neoplasms diagnosed in the Department of Morbid Anatomy and Forensic Medicine OAUTHC, Ile-Ife from 1st July 1996 to 30th June 2016.
Variables
We noted the age, sex of the patients, nature of histological diagnosis (benign and malignant) and the specific histologic diagnosis. We evaluated HBME-1 immunostain grade (0, 1+, 2+, 3+, 4+) for all the tumours. We scored the HBME-1 staining grade for each case without knowledge of the histologic diagnosis to reduce bias. We graded the tumours based on the percentage of tumour cells on the histological slides that were positive for the HBME-1 antibody. HBME-1 expression was evaluated thus:
0: staining of < 10% of tumour cells
1+: staining of 10% - 25% of tumour cells
2+: staining of 26% - 50% of tumour cells
3+: staining of 51% - 75% of tumour cells
4+: staining of > 75% of the tumour cells
Score of zero was classified as negative, score of 1+ or 2+ was considered as focal staining and 3+ or 4+ was viewed as diffuse staining.12
Data sources/measurement
We used heat-induced epitope retrieval method for antigen retrieval. We dipped the fixed slides in citrate buffer (pH=6.0) diluted with distilled water (1:10) and then transferred the slides to a microwave. We set the temperature of the microwave to 500C and then gradually increased it to 900C. We moved the slides from the microwave to a fresh citrate for 20 minutes for cooling and then dipped the slides in Phosphate Buffer solution (PBS).
We subjected all the tissue block samples to HBME-1 antibody (Dako M3505). We ensured the veracity of each biochemical reaction by using positive and negative controls. We used normal tracheal epithelium as the positive control as specified in the manufacturer’s manual.
We placed the slides in a humidity chamber and added 3% hydrogen peroxide to each slide for 10 minutes. We rinsed the slides in 0.1% PBS. We then incubated the specimens with 40-130 µl of an appropriately characterized and diluted monoclonal HBME-1 antibody at 60oC for one hour. We followed this step by incubating with an undiluted labelled polymer Horse Radish Peroxidase (HRP) and conjugated anti-mouse secondary antibody for 30 minutes. We rinsed the slides with PBS and then wiped the areas surrounding the tissue dry. We then added 1ml of Diaminobenzidine solution (Catalogue number TA-125-QHDX) to cover the specimen. We incubated the slide covered by Diaminobenzidine in a humidity chamber for 15 minutes.
We immersed the slides in a bath of aqueous haematoxylin about ten times and then rinsed them in distilled water for 3 minutes. We passed the slides through a series of graded alcohol to dehydrate the tissue (70%, 95% then 100%). We then rinsed the tissue with xylene. We applied a mounting fluid on the slide and then placed a cover slip over it. We evaluated the slides using a Leica 500 binocular microscope and graded the stains using the grading scheme earlier outlined.12
Study Size and Bias
We studied all thyroid neoplasm samples seen during the study period. Cases with missing data or tissue blocks were excluded from the study. We did not do any sampling.
Statistical Methods
Statistical analysis was done using Microsoft excel and Statistical Package for Social Sciences (SPSS) version 20. The data were presented as tables. The Chi square test was done with level of significance (p value) set at 0.05. Sensitivity and specificity were determined using the standard formula.
Ethical Consideration
The study was approved by the Ethics and Research Committee of OAUTHC, Ile-Ife.
Results
During the 20-year period of study, 31,501 surgical specimens were received at the Department of Morbid Anatomy and Forensic Medicine, OAUTHC Ile-Ife. Of these, 497 cases (1.58% of the total specimens received during the period of study) were thyroid gland specimens and 80 (16.1% of the total thyroid cases) of the thyroid specimens were thyroid neoplasms. We excluded twenty-four (30%) cases because we could not retrieve adequate tissues for the study. We studied only fifty-six cases (all neoplastic thyroid diseases). Sixteen (28.6%) and forty (71.4%) cases of the cases were benign and malignant, respectively. The Malignant thyroid neoplasms were 2.5 times more frequent than the benign thyroid lesions. The sex distribution of the benign and malignant cases is shown in table 1.
Table 1: Combined sex distribution of both benign and malignant thyroid neoplasms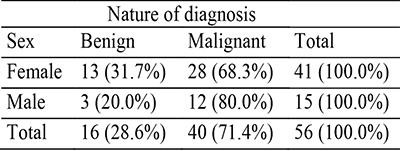
General expression HBME-1
HBME-1 showed a predominantly membranous pattern, however in some cases there was luminal staining. Twelve of the neoplastic cases were negative for HBME-1 and forty-four showed varying levels of positivity. Eleven of the cases showed focal staining of the tumour cells and thirty-three showed diffuse staining of the tumour cells. There is a significant statistical relationship between HBME-1 expression and the nature of the neoplastic cases (p = 0.001). Table 2 shows HBME-1 pattern of staining among the neoplastic thyroid cases
Table 2: HBME-1 staining distribution among the thyroid tumours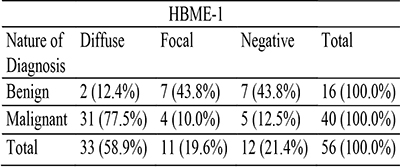
Expression of immunomarkers in benign thyroid neoplasm
There were sixteen benign thyroid neoplasms in this study, all of which were follicular adenomas. Seven (43.8%) patients with follicular adenoma showed no expression of HBME-1 while the remaining nine (56.2%) displayed varying degrees of HBME-1 expression. Seven (43.8%) of the remaining cases of follicular adenoma showed focal expression and two (12.4%) showed diffuse expression of HBME-1. Fourteen (87.6%) of the benign thyroid neoplasm showed either no expression or focal expression of HBME-1. The ratio of negative and focal staining of follicular adenoma to diffuse staining was 7.1:1. The sensitivity of HBME-1 in distinguishing benign from malignant thyroid neoplasms in this study was 77.5%, the specificity was 87.5%, the positive predictive value (PPV) was 93.3% and the negative predictive value (NPV) was 60.1%. Table 3 shows expression of HBME-1 in all the study cases.
Table 3: Expression of HBME-1 in the neoplastic thyroid cases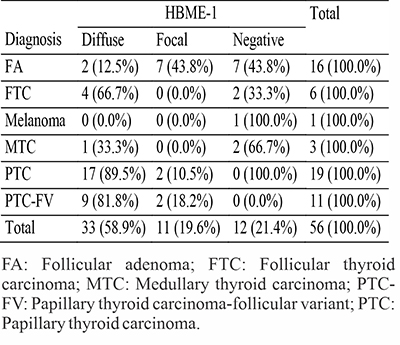
Expression of immunomarkers in malignant thyroid neoplasm
Five (12.5%) of patients with malignant thyroid neoplasm showed no expression of HBME-1 while the remaining thirty-five (85.0%) displayed varying degrees of HBME-1 expression. Four (10.0%) of the cases showed focal expression and the rest (75.0%) showed diffuse expression of HBME-1. Therefore, nine (22.5%) of the malignant cases showed either focal or no expression of HBME-1.
All the thirty PTCs (19 classic PTCs and 11 follicular variant of PTCs) showed positive staining for HBME-1, four (two classic PTC and two PTC-FV) were focal and twenty-six (17 classic PTCs and 9 follicular variant of PTCs) were diffusely positive. Two of the FTCs were negative for HBME-1 and four showed diffuse expression of the immunomarker. Two MTCs cases were negative for the expression of HBME-1. The solitary case of melanoma was negative for the HBME-1. Figure 1-6 shows photomicrographs of haematoxylin and eosin stains, and HBME-1 immunohistochemistry of typical cases of thyroid carcinomas seen in our study.
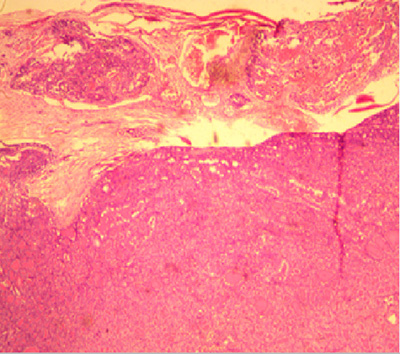
Figure 1: Photomicrograph showing diffuse proliferation of sheet of follicular cells with attempt at splitting the capsule and vascular invasion by the tumour cells in a case diagnosed to be follicular carcinoma in a 30-year-old male (H&E x 200).
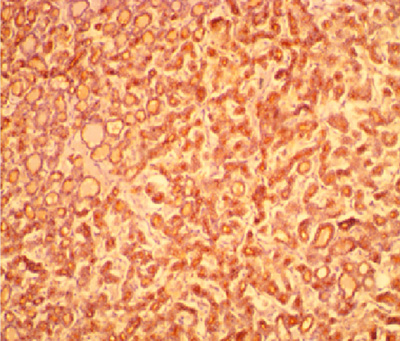
Figure 2: Photomicrograph of the same patient above showing diffuse staining of the cells lining the micro and macrofollicles by HBME-1 (x 200).
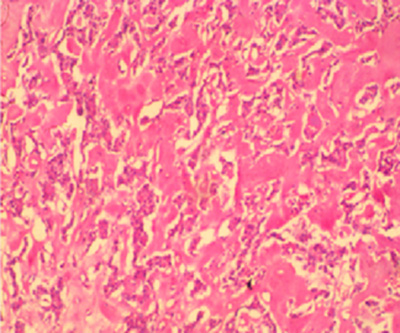
Figure 3: Photomicrograph showing variably sized and shaped nests of cells in a background of amorphous acellular eosinophilic material in a case of medullary carcinoma in a 36-year-old woman (H&E x200).
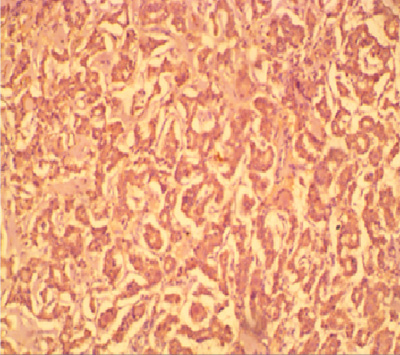
Figure 4: Photomicrograph of the same Figure 2 patient above showing diffuse staining of tumour cells by HBME-1. (x 200)
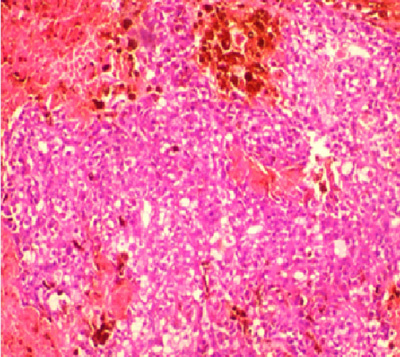
Figure 5: Photomicrograph showing proliferating cluster of malignant melanin-producing melanocytes in a case of melanoma in a 47-year-old female (H&E x 400).
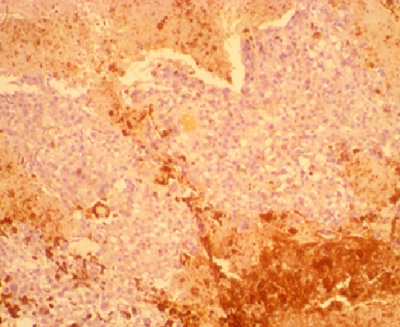
Figure 6: Photomicrograph of the same patient above showing no staining of the malignant melanocytes by HBME-1. The brown staining areas are melanin pigment, the cytoplasm of the melanocytes is free of this staining. HBME-1 x 400.
Discussion
Our study suggests that HBME-1 can be an added tool in distinguishing follicular adenoma from other malignant thyroid tumours. Most of the follicular adenomas in this study do not show diffuse expression of HBME-1. Out of all the sixteen benign neoplasms which are all follicular adenomas only 12.4% showed diffuse expression of HBME-1. The rest of the follicular adenomas (87.6%) showed either no expression or focal expression. Our finding is similar to that of the work done by Prasad et al, where 10.0% of the follicular adenomas were positive for HBME-1.11 Scognamiglio13 and others in New York recorded a lower percentage (4%) of follicular adenomas positive for HBME-1. Nechifor-Boila et al14 and Park et al15 observed higher values of 40% and 48.6% respectively.
The HBME-1 staining pattern in follicular adenoma suggests that follicular lesions of the thyroid that stain strongly and diffusely with HBME-1 are likely to be malignant. This can help to differentiate follicular carcinoma and follicular variant of papillary thyroid carcinoma from follicular adenoma. HBME-1 staining is probably not useful in differentiating follicular adenoma from non-neoplastic follicular thyroid lesions and normal thyroid tissue as a relatively high percentage of follicular adenomas are seen not to be stained by the antibody or to have a focal staining that is commonly associated with the latter group of lesions.
The overall sensitivity and specificity of HBME-1 for thyroid malignancies in this study were 77.5% and 87.5% respectively. These are comparable with findings of Liu and colleagues that got 78.8% sensitivity and 82.1% specificity for thyroid malignancies.7 The relatively high sensitivity and specificity supports its use in conjunction with other antibodies as suggested by other authors. Our study also negates its use as a single antibody in identification of malignant lesions since the sensitivity and specificity is not up to 100%.
Our work suggests that HBME-1 negative thyroid tumours are not likely to be papillary thyroid carcinomas or follicular variant of papillary thyroid carcinoma. In our study, all cases of papillary thyroid carcinomas were positive for HBME-1 with the overwhelming majority (86.7%) of papillary thyroid carcinomas showing strong and diffuse expression of HBME-1. Our finding is like the observation of Prasad et al11 that reported 85.0% positive expression amongst papillary thyroid carcinomas. Park and others15 noted a slightly higher value of 91.7% in Seoul. We recommend that pathologists should be wary of diagnosing a papillary thyroid carcinoma in HBME-1 negative tumours. The clinical and routine histological features should be considered for every case. These should be interpreted along with other immunophenotypic characteristics of the tumour.
A focal or negative staining does not exclude a malignant follicular lesion. In our study, 66.7% of FTCs diffusely expressed HBME-1 while 33.3% were negative for the immunomarker. This observation is in agreement with the finding of Saleh et al that documented 64.0% HBME-1 expression in follicular carcinomas.16 Park and colleagues got a lower value (50.0%).15
Medullary thyroid carcinomas are generally known to be negative for HBME-1 antigens.17 This is expected as they are believed to originate from parafollicular cells, derivatives of neural crests. One of the medullary thyroid carcinomas showed diffuse staining pattern of HBME-1. The other two medullary thyroid carcinomas were negative for HBME-1. Our finding show that medullary thyroid carcinomas can be positive for HBME-1. Depending on the nature of the case, it might be necessary to do ancillary investigations like Congo red stain for extracellular amyloid material, and S100 and chromogranin immunohistochemistry to confirm the diagnosis. It is, however, important to note that owing to the rarity of medullary thyroid carcinoma, the number of cases used in many studies including our study is small. A multi-centered study with pooling together of large numbers of medullary thyroid carcinoma cases may give more statistically comprehensive findings and recommendations.
HBME-1 antibody may be useful in differentiating thyroid carcinomas from melanomas in metastatic carcinomas. This may be particularly useful since both thyroid cancers and melanomas are known to commonly spread to the bones. The sole case of melanoma in this study was negative for HBME-1. A HBME-1 positive metastatic tumour may likely be from a thyroid carcinoma than a melanoma. A study of HBME-1 expression in melanomas and other neural crest derivatives will help to evaluate its use in differential diagnosis of metastatic cancers.
Although we used controls to improve the accuracy and reliability of our study, it is still partly limited by using archival tissue blocks which may not have optimally preserved antigens.
Conclusion
HBME-1 immunophenotype of the neoplastic cases in the study has been described and a significant statistical association between HBME-1 and the nature of thyroid neoplasms: benign or malignant has been reported.
Strong and diffuse staining of tumour cells favors a malignant lesion. Interpretation of HBME-1 immunohistochemistry in thyroid neoplasms calls for caution because focal positive staining could occur in benign neoplastic cases and very rarely in non-neoplastic lesions. It is, however, important to note that immunohistochemistry is a supplementary procedure that helps in equivocal cases. It is not a replacement for haematoxylin and eosin histomorphology interpretation.
References
- Maitra A. The Endocrine System. In: Kumar V, Abbas A, Aster J, editors. Robbins and Cotran Pathologic Basis of Disease. 9th ed. Philadelphia, PA: Elsevier Saunders; 2015. p. 1082–100.
- Tsegaye B, Ergete W. Histopathologic pattern of thyroid disease. East Afr Med J. 2003 Oct;80(10):525–8.
- Ahmed SA, Rafindadi AH, Iliyasu Y, Shehu SM. Patterns of thyroid cancers in Zaria. Highland Medical Research Journal. 2007;5(2):7–10.
- Der EM, Quayson SE, Clegg-Lamptey JN, Wiredu EK, Ephraim RKD, Gyasi RK. Thyroid Disorders in Accra, Ghana: A Retrospective Histopathological Study at the Korle-Bu Teaching Hospital. Journal of Medical and Biomedical Sciences [Internet]. 2013 Apr 8 [cited 2023 May 2];2(1). Available from: https://www.ajol.info/index.php/jmbs/article/view/87146
- Ariyibi OO, Duduyemi BM, Akang EE, Oluwasola AO. Histopathological Patterns of Thyroid Neoplasms in Ibadan Nigeria: A Twenty Year Retrospective Study. International Journal of TROPICAL DISEASE & Health. 2013 Apr 2;148–56.
- Morris LGT, Sikora AG, Tosteson TD, Davies L. The increasing incidence of thyroid cancer: the influence of access to care. Thyroid. 2013 Jul;23(7):885–91.
- Liu H, Lin F. Application of immunohistochemistry in thyroid pathology. Arch Pathol Lab Med. 2015 Jan;139(1):67–82.
- Fischer S, Asa SL. Application of immunohistochemistry to thyroid neoplasms. Arch Pathol Lab Med. 2008 Mar;132(3):359–72.
- Rezk S, Khan A. Role of immunohistochemistry in the diagnosis and progression of follicular epithelium-derived thyroid carcinoma. Appl Immunohistochem Mol Morphol. 2005 Sep;13(3):256–64.
- Fusco A, Chiappetta G, Hui P, Garcia-Rostan G, Golden L, Kinder BK, et al. Assessment of RET/PTC oncogene activation and clonality in thyroid nodules with incomplete morphological evidence of papillary carcinoma: a search for the early precursors of papillary cancer. Am J Pathol. 2002 Jun;160(6):2157–67.
- Prasad ML, Pellegata NS, Huang Y, Nagaraja HN, de la Chapelle A, Kloos RT. Galectin-3, fibronectin-1, CITED-1, HBME1 and cytokeratin-19 immunohistochemistry is useful for the differential diagnosis of thyroid tumors. Mod Pathol. 2005 Jan;18(1):48–57.
- Nechifor-Boilă A, Cătană R, Loghin A, Radu Tg, Borda A. Diagnostic value of HBME-1, CD56, Galectin-3 and Cytokeratin-19 in papillary thyroid carcinomas and thyroid tumors of uncertain malignant potential. Rom J Morphol Embryol. 2014;55(1):49–56.
- Scognamiglio T, Hyjek E, Kao J, Chen YT. Diagnostic usefulness of HBME1, galectin-3, CK19, and CITED1 and evaluation of their expression in encapsulated lesions with questionable features of papillary thyroid carcinoma. Am J Clin Pathol. 2006 Nov;126(5):700–8.
- Nechifor-Boilă A, Cătană R, Loghin A, Radu TG, Borda A. Diagnostic value of HBME-1, CD56, Galectin-3 and Cytokeratin-19 in papillary thyroid carcinomas and thyroid tumors of uncertain malignant potential. Rom J Morphol Embryol. 2014;55(1):49–56.
- Park YJ, Kwak SH, Kim DC, Kim H, Choe G, Park DJ, et al. Diagnostic value of galectin-3, HBME-1, cytokeratin 19, high molecular weight cytokeratin, cyclin D1 and p27(kip1) in the differential diagnosis of thyroid nodules. J Korean Med Sci. 2007 Aug;22(4):621–8.
- Saleh HA, Jin B, Barnwell J, Alzohaili O. Utility of immunohistochemical markers in differentiating benign from malignant follicular-derived thyroid nodules. Diagn Pathol. 2010 Jan 26;5:9.
- Palo S, Biligi DS. Differential diagnostic significance of HBME-1, CK19 and S100 in various thyroid lesions. Malays J Pathol. 2017 Apr;39(1):55–67.